Hepatit B aşısının yan etkileri hakkında itiraf
Aşıların yoğun tartışıldığı bugünlerde Prof Dr Ahmet Rasim Küçükusta, aşı firmalarının kendi belgeleriyle vurdu. İşte Hepatit B ile ile o yazı ve belge
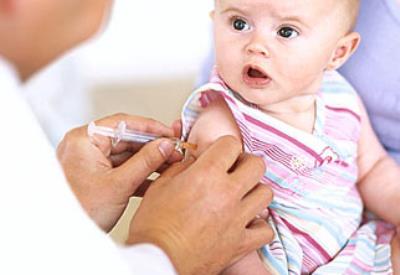
Bilim dünyası, çocuklara mutlaka yapılması tavsiye edilen aşıların hiçbir yan etkisi olmadığını, bunların güvenle yapılabileceğini iddia ediyor.
Gelin, bunlar içinden halk arasında hepatit B aşısı olarak bilinen ve ilk dozu her bebeğe dünyaya gelir gelmez uygulanan aşının üretici firma tarafından yayınlanan prospektüsünden 6. bölümü okuyalım.
Şu hususlar dikkatimi çekti:
BİR: Aşının bebek ve 10 yaşına kadar olan 147 çocuğa uygulandığı ve bunların her dozdan sonra sadece 5 gün takip edildiği anlaşılıyor.
147′ den kaçının bebek olduğu bile belli olmayan üç çalışmaya dayalı “istenmeyen etki” değerlendirmesinin ne kadar sağlıklı olacağının takdirini size bırakıyorum.
İKİ: Aşı piyasaya çıktıktan sonra bildirilen “istenmeyen tesirler” içinde oto-immün hastalıklar, MS, havale, miyelit, artrit, trombositopeni, optik nörit ve daha sayısız pek çok ciddi hastalıklar var.
ÜÇ: Prospektüsün 13.1 bölümünde de aşının “karsinojenik veya mutajenik potansiyeli veya fertiliteyi bozma potansiyeli değerlendirilmemiştir” ifadesi de çok dikkat çekiyor.
Bu, aşının kanser, genlerde mutasyon ve kısırlık yapıp yapmadığının belli olmadığı manasına geliyor.
Merck firması tarafından üretilen RECOMBIVAX HB isimli aşının ürün prospektüsünde “advers reaction” yani aksi tesirler (olumsuz ilaç reaksiyonları) bölümü (1):
6. ADVERSE REACTIONS
In healthy infants and children (up to 10 years of age), the most frequently reported systemic adverse reactions (>1% injections), in decreasing order of frequency, were irritability, fever, diarrhea, fatigue/weakness, diminished appetite, and rhinitis. In healthy adults, injection site reactions and systemic adverse reactions were reported following 17% and 15% of the injections, respectively.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
In three clinical studies, 434 doses of RECOMBIVAX HB, 5 mcg, were administered to 147 healthy infants and children (up to 10 years of age) who were monitored for 5 days after each dose.
Injection site reactions and systemic adverse reactions were reported following 0.2% and 10.4% of the injections, respectively.
The most frequently reported systemic adverse reactions (>1% injections), in decreasing order of frequency, were irritability, fever (³101°F oral equivalent), diarrhea, fatigue/weakness, diminished appetite, and rhinitis.
In a study that compared the three-dose regimen (5 mcg) with the two-dose regimen (10 mcg) of
RECOMBIVAX HB in adolescents, the overall frequency of adverse reactions was generally similar.
In a group of studies, 3258 doses of RECOMBIVAX HB, 10 mcg, were administered to 1252 healthy adults who were monitored for 5 days after each dose.
Injection site reactions and systemic Adverse reactions were reported following 17% and 15% of the injections, respectively.
The following Adverse reactions were reported: Incidence Equal To or Greater Than 1% of Injections
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
Injection site reactions consisting principally of soreness, and including pain, tenderness, pruritus, erythema, ecchymosis, swelling, warmth, nodule formation.
The most frequent systemic complaints include fatigue/weakness; headache; fever (≥100°F); malaise.
GASTROINTESTINAL DISORDERS
Nausea; diarrhea
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS
Pharyngitis; upper respiratory infection
Incidence Less Than 1% of Injections
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
Sweating; achiness; sensation of warmth; lightheadedness; chills; flushing
GASTROINTESTINAL DISORDERS
Vomiting; abdominal pains/cramps; dyspepsia; diminished appetite
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS
Rhinitis; influenza; cough
NERVOUS SYSTEM DISORDERS
Vertigo/dizziness; paresthesia
SKIN AND SUBCUTANEOUS TISSUE DISORDERS
Pruritus; rash (non-specified); angioedema; urticaria
MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS
Arthralgia including monoarticular; myalgia; back pain; neck pain; shoulder pain; neck stiffness
BLOOD AND LYMPHATIC DISORDERS
Lymphadenopathy
PSYCHIATRIC DISORDERS
Insomnia/disturbed sleep
EAR AND LABYRINTH DISORDERS
Earache
RENAL AND URINARY DISORDERS
Dysuria
CARDIAC DISORDERS
Hypotension
6.2 Post-Marketing Experience
The following additional adverse reactions have been reported with use of the marketed vaccine.
Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to a vaccine exposure.
Immune System Disorders
Hypersensitivity reactions including anaphylactic/anaphylactoid reactions, bronchospasm, and urticaria have been reported within the first few hours after vaccination.
An apparent hypersensitivity syndrome (serum-sickness-like) of delayed onset has been reported days to weeks after vaccination, including:
arthralgia/arthritis (usually transient), fever, and dermatologic reactions such as urticaria, erythema multiforme, ecchymoses and erythema nodosum [see Warnings and Precautions (5.1)].
Autoimmune diseases including systemic lupus erythematosus (SLE), lupus-like syndrome, vasculitis, and polyarteritis nodosa have also been reported.
Gastrointestinal Disorders
Elevation of liver enzymes; constipation
Nervous System Disorders
Guillain-Barré syndrome; multiple sclerosis; exacerbation of multiple sclerosis; myelitis including transverse myelitis; seizure; febrile seizure; peripheral neuropathy including Bell's Palsy; radiculopathy; herpes zoster; migraine; muscle weakness; hypesthesia; encephalitis
Skin and Subcutaneous Disorders
Stevens-Johnson syndrome; alopecia; petechiae; eczema
Musculoskeletal and Connective Tissue Disorders
Arthritis
Pain in extremity
Blood and Lymphatic System Disorders
Increased erythrocyte sedimentation rate; thrombocytopenia
Psychiatric Disorders
Irritability; agitation; somnolence
Eye Disorders
Optic neuritis; tinnitus; conjunctivitis; visual disturbances; uveitis
Cardiac Disorders
Syncope; tachycardia
The following adverse reaction has been reported with another Hepatitis B Vaccine (Recombinant) but not with RECOMBIVAX HB: keratitis.
Kaynak:
- http://www.merck.com/product/usa/pi_circulars/r/recombivax_hb/recombivax_pi.pdf













Yorum Yap